Peripheral neuromodulation is emerging as one of the most promising strategies in modern medicine. It is based on the application of electrical stimuli to peripheral nerves to modulate the activity of the nervous system. Its potential benefits include, among others, the management of chronic pain, inflammatory modulation, and neurological rehabilitation. However, an essential question arises: what does current scientific evidence tell us?

How does peripheral neuromodulation work?
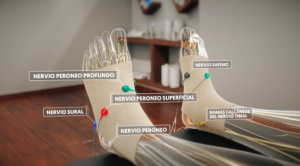
Peripheral nerves connect the body with the brain. When injury or chronic disease occurs, these circuits can become dysregulated and hyperactive, leading to persistent pain, inflammation, or loss of function.
Peripheral neuromodulation acts as a regulator of this activity. Some mechanisms proposed in the scientific literature include:
- Activation of non-nociceptive Aβ sensory fibres, which can compete with pain signals and reduce their transmission.
- Modulation of the immune system, with a decrease in pro-inflammatory cytokines in certain experimental models.
- Autonomic balance, improving communication between the sympathetic and parasympathetic systems.
- Neural plasticity stimulation, promoting the reorganisation of neural connections after injury or prolonged stimulation.
Scientific evidence: where do we already have results?
Below is a summary of some fields in which peripheral neuromodulation has shown relevant clinical outcomes:
- Neuropathic pain: In postherpetic neuralgia and neuropathic pain in general, peripheral nerve stimulation has demonstrated significant analgesic effects in multiple clinical trials and systematic reviews.
- Chronic low back pain: Clinical reviews recommend peripheral neuromodulation as an adjuvant option for patients with refractory chronic low back pain, although study designs remain heterogeneous.
- Postoperative pain and phantom limb syndrome: Randomised trials show that percutaneous nerve stimulation applied during the weeks following amputation or surgery can reduce phantom limb pain and improve mobility in certain patients.
- Chronic inflammatory diseases: Vagal stimulation (both invasive and non-invasive) activates he cholinergic anti-inflammatory pathway, reducing markers such as TNF-α and IL-6 in experimental and clinical studies on rheumatoid arthritis and and Crohn’s disease..
The role of the vagus nerve: beyond the periphery
Although it belongs to the peripheral nervous system, the vagus nerve directly connects the organs, immune system and brain.
Its stimulation is considered a bridge between local and systemic neuromodulation, with a global impact on the body.
Non-invasive vagal stimulation (nVNS) devices are already approved for the treatment of migraine and cluster headache, highlighting the therapeutic potential of this axis.
Where does NESA® fit into this scenario?
From a conceptual standpoint, there are two main approaches within peripheral neuromodulation:
- Local intervention, directed at specific nerves for the treatment of localised conditions (such as chronic or postoperative pain).
- Systemic intervention, aimed at modulating autonomic and inflammatory networks, influencing functions such as sleep, emotional balance and overall recovery.
The NESA® XSIGNAL technology is positioned within this second approach. Through gentle, non-invasive electrical stimulation applied at distal points of the body, it seeks to modulate the autonomic nervous system globally.
Potential effects include:
- Contributing to the restoration of sympathetic–parasympathetic balance.
- Reducing inflammatory hyperactivity associated with chronic pain and fatigue.
- Supporting neurological and functional recovery, according to currently available clinical data.
In this way, NESA® represents the clinical application of the latest advances in peripheral neuromodulation, offering an innovative approach to autonomic regulation and functional recovery, with a well-documented safety profile and a rapidly expanding field of evidence.

NESA®: Current Perspectives and Future Directions
Medicine and physiotherapy are moving towards personalised treatments, with less invasive devices and protocols tailored to each patient.
The main objective is to contribute to the management of complex conditions through solutions grounded in evidence, safety, and scientific rigour.
Reference
Bonaz, B., Sinniger, V., Pellissier, S., & Picq, C. (2021). Vagus nerve stimulation at the interface of brain–gut interactions in inflammatory bowel disease and rheumatoid arthritis. Nature Reviews Gastroenterology & Hepatology, 18(3), 161–177. https://doi.org/10.1038/s41575-020-00388-7
Dawson, J., Liu, C. Y., Francisco, G. E., Cramer, S. C., Wolf, S. L., Dixit, A., … & Durand, D. M. (2021). Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, device trial. The Lancet, 397(10284), 1545–1553. https://doi.org/10.1016/S0140-6736(21)00475-X
Deer, T. R., et al. (2022). Evidence-based consensus guidelines for peripheral nerve stimulation. Pain Physician, 25(3), E305–E324.
Deer, T. R., Pope, J. E., Lamer, T. J., Provenzano, D. A., FitzGerald, J. J., Hunter, C. W., & Mekhail, N. (2024). Peripheral nerve stimulation for the treatment of chronic neuropathic pain: Current perspectives and future directions. Pain and Therapy, 13(2), 351–367. https://doi.org/10.1007/s40122-024-00659-6
Gilmore, C. A., Deer, T. R., Desai, M. J., et al. (2025). Four-year follow-up from a prospective, multicentre study of percutaneous 60-day peripheral nerve stimulation for chronic low back pain. Pain and Therapy, 14, 1103–1115. https://doi.org/10.1007/s40122-025-00737-3
Gilmore, C. A., Kapural, L., McGee, M. J., Boggs, J. W., & Rosen, A. (2022). Peripheral nerve stimulation for chronic low back pain: A prospective multicentre trial. Pain Medicine, 23(7), 1223–1234. https://doi.org/10.1093/pm/pnab329
Goadsby, P. J., Grosberg, B. M., Mauskop, A., Cady, R., & Simmons, K. A. (2014). Non-invasive vagus nerve stimulation for acute treatment of cluster headache: The PREVA study. Cephalalgia, 34(12), 986–999. https://doi.org/10.1177/0333102414557899
Koopman, F. A., Chavan, S. S., Miljko, S., Grazio, S., Sokolović, S., Schuurman, P. R., Mehta, A. D., Levine, Y. A., Faltys, M., Zitnik, R., Tracey, K. J., & Tak, P. P. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proceedings of the National Academy of Sciences, 113(29), 8284–8289. https://doi.org/10.1073/pnas.1605635113
Rao, R., Zhou, Y., D’Souza, R. S., & Bendel, M. A. (2021). Percutaneous peripheral nerve stimulation for postoperative and phantom limb pain: A systematic review. Pain Reports, 6(6), e972. https://doi.org/10.1097/PR9.0000000000000972
Rauck, R. L., et al. (2021). Percutaneous peripheral nerve stimulation for the treatment of post-amputation pain. Pain Medicine, 22(3), 548–560. https://doi.org/10.1093/pm/pnaa430
Sio, L. C. O., Hom, B., Garg, S., & Abd-Elsayed, A. (2023). Mechanism of action of peripheral nerve stimulation for chronic pain: A narrative review. International Journal of Molecular Sciences, 24(5), 4540. https://doi.org/10.3390/ijms24054540